Interested in Element Collecting?
Collecting things has always been the proving ground that separates the mere dabbler from the true enthusiast. First-edition books, baseball cards, comic books, battle card games like Magic: The Gathering and Yu-Gi-Oh!, rare coins, and memorabilia are just a few of the more common collectible and well-known examples. For the hardcore chemistry connoisseur, nothing could be more exciting to collect than the building blocks of our reality: the elements of the periodic table themselves!
Until recently, element collecting was a difficult, prohibitively expensive, and time-consuming hobby with mixed results and dubious sample purity. Some of the more exotic elements, such as uranium and cesium, have been very difficult by ordinary people. Others are too unstable and/or dangerous to capture in their elemental form without specialized equipment such as chlorine or thallium.
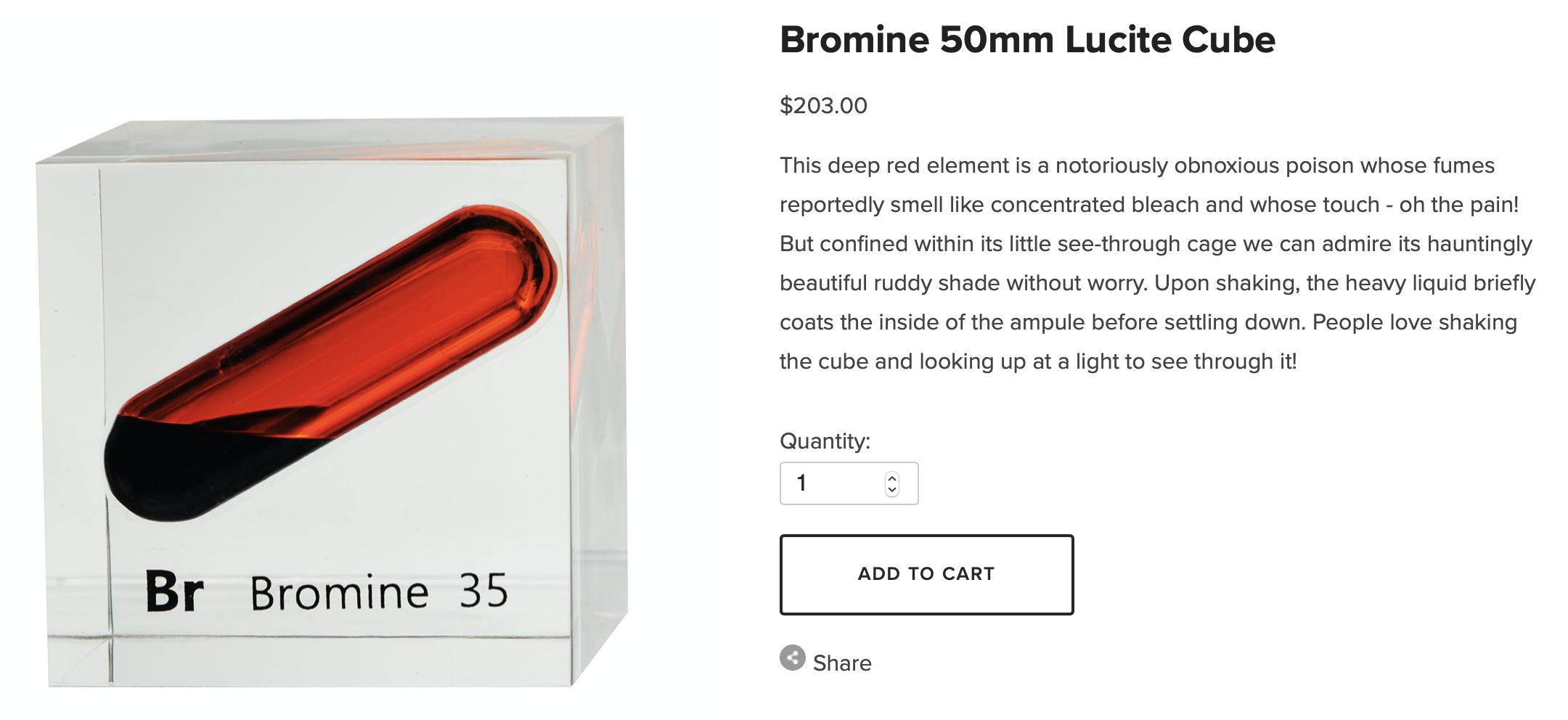
Many elements are problematic for collectors. Some are radioactive, throwing off alpha particles, beta radiation, and gamma rays which can be harmful to humans as they decay into more stable forms. Some, like mercury and arsenic, are dangerous to touch with bare hands. Some elements react violently to the presence of or contact with water or oxygen. And what of the gas elements? Storing them in purpose-built glass ampules is the only sensible choice.
For many years, collectors got around these limitations by displaying household items related to the elements which they had collected, rather than pure examples of the collected elements themselves.
For example, they might have displayed a spoonful of salt as a stand-in for sodium (and chlorine!) since neither was easily available in elemental form. Pepto-Bismol could be a stand-in for bismuth and Milk of Magnesia for magnesium. Interestingly, the “lead” in pencils has been another age-old popular workaround which, ironically, was a misnomer since they’re carbon…. another element entirely!
Argon, krypton, neon, and xenon could be represented by neon signs incorporating these luminescent noble gases, which fluoresce different colors when an electrical current is passed through them, exciting the atoms. Tantalum capacitors, neodymium magnets, smoke detectors with the tiniest dab of radioactive americium, and phosphorus flashbulbs are some other common examples of items that might be displayed in this sort of collection of elements.
The downside - and simultaneous dismay - for the purists in the collecting community has been that all of these workarounds have come at the expense of purity. In some cases pure options presented huge challenges while in others they were simply insurmountable.
Luciteria was born to cater to the unmet needs of these collectors and to make element collecting safer, more enjoyable, more eye-catching, and more fun than ever before!
Collecting chemical elements of nearly perfect purity used to be the sole province of military-industrial research, museum, and university physics departments with nearly unlimited resources and specialized laboratory space. Most casual users and avid collectors alike could only collect elements confined to the contents of a commercially available chemistry set. These elements were often compounds, such as manganese dioxide, not pure nickel or pure silicon. This in turn placed the ability to buy more exotic elements such as plutonium and scandium well beyond the reach of the average high school chemistry teacher or parent wanting to teach their children about the wonders of the natural world, as the mineral forms were often hideously expensive, adulterated or both.
By embedding representative samples of the elements in Lucite acrylic, they can be displayed and handled safely, without risk to the owner or the appearance of the sample. Even better, the cubes display the elements of the periodic table complete with their name, symbol, and atomic number, making them a portable and exciting learning tool.
With both the embedded samples cast in Lucite acrylic and solid cubes of all the metallic elements, collecting elements in ultrapure form does away with the disappointment of incomplete and/or unsafe “witches’ brew” collections. With a minimum of 99% purity, and typically far higher, purists and casual collectors alike can easily enjoy the natural beauty of the building blocks of our universe.
The Lucite cubes can be displayed on their own, for example as a desk ornament or paperweight, or in our specialty display cases. Each sample is attractively displayed in a form that is often unique and always visually impacting manner. The blocks themselves are virtually indestructible with normal or even extreme handling. It would take a dedicated effort and power tools to break them apart; making them ideal even for element collectors with butterfingers. Even in the case of radioactive elements the samples are typically microscopic and the shielding provided by the outer acrylic shell is sufficient to eliminate any radiation from being detectable from the outside.
Before Luciteria, the options for private collectors to buy elements were very limited. Obtaining complete sets of the elements which were stable and safe for display while assuring purity was a painstaking, time-consuming affair that often thwarted the efforts of even the most dedicated, and patient of enthusiasts. Luciteria’s elements cubes are sourced directly from laboratories to exacting specifications allowing for a much wider community to acquire samples that are attractive, affordable and safe to own and display!
Who collects elements samples anyway?
Anyone can be an element collector. Pioneering neurosurgeon Dr. Oliver Sacks, portrayed by legendary comedian and actor Robin Williams alongside Robert DeNiro in the movie Awakenings based on Sacks’ eponymous book, was an avid elements enthusiast who would often receive periodic table element samples from friends and colleagues around the world. In fact, he was such an avid proponent of this hobby that he was often known to refer to his age by the element whose atomic number corresponded to it. In an article for the Atlantic, he mentioned receiving a “charming box” from a British friend, inscribed with the sentiment, “Happy Thallium Birthday,” which would have made him 81 at the time. (He passed away at age 82.)
Justin Urgitis, a former employee of pharmaceutical giant Pfizer who is now the data operations lead at Syneos Health, started his collection in college while pursuing a degree in forensic chemistry as a result of an interest stirred in high school science class. He started his collection with cobalt, iodine, magnesium, and aluminum, and is known to joke, “Want to come upstairs and see my barium?” riffing on the classic pick-up line.
Everyday people from all walks of life can enjoy element collecting. Science buffs, engineers, physicists, chemistry majors, attorneys, writers, and homemakers all seek out pure forms of elements for reasons ranging from pure enjoyment to teaching tools. And who doesn’t like having some precious metals around the house or office to impress visitors? Bismuth crystals, with their distinctive rainbow coloration, make an interesting conversation piece for a desk or seating area, while pure gold, a mineral sample or pure silver coin can create a powerful statement of taste and prestige. Mercury in its liquid metal form is always a crowd-pleaser.
All of Luciteria’s elements cubes are backed by our 100% satisfaction guarantee, for the best representative element collecting possible.
Each element’s sample we create is individually assayed to assure the highest possible purity and quality standards. Our virtually indestructible acrylic cubes are guaranteed not to discolor with proper care and handling, and will not yellow or cloud over time.
Whether you want to purchase a mineral sample of carbon, an eye-catching zirconium metal display or to finally get that elusive niobium metal sample you’ve been searching for, Luciteria has you covered. Check out our comprehensive list of currently available elements at the bottom of this page, or click here to explore our currently available inventory and start an elements collection of your own today!
Frequently Asked Questions About Element Collecting
Question: If I store collected elements in the periodic table display board you offer, will that affect the centerpiece quality of the board itself? --Stephen, Rapid City, SD
Answer: Not at all! That’s what the board is for. In fact, we think, just like the universe itself, the periodic table looks better when it’s filled with all the elements. Your visitors and students will enjoy it a great deal more, and you’ll love how much more fun and interactive you can make science classes when you can actually allow people to hold the elements you’re talking about in their hands!
Question: Wouldn’t an actual nickel be the best sample of the element nickel? --Erica, Las Vegas, NV
Answer: US nickels have been alloys of 75% copper and 25% nickel since 1866. From 1942 to 1945, nickels were made of 35% silver. These nickels, recognized by the letters “D”, “P” or “S” over the Monticello dome, are worth more than their face value and are very rare nowadays, as they have been largely taken out of circulation. Much like most iron and steel products, which are not pure iron but a combination of iron and nickel or iron and carbon, these are not pure elemental representations. If you want pure, elemental nickel, Luciteria can provide it in mineral or reference density block form, among others!
Question: Can I safely display reactive gases like fluorine and chlorine? --Matilda, Essex, UK
Answer: With Luciteria’s Lucite element blocks, you absolutely can! All our gas samples are stored in vacuum ampules prior to embedding in the acrylic matrix. This means, unless you make a dedicated effort to break the block using a hacksaw or power tools, the display is perfectly safe and harmless to handle.
Question: Why do people think we should celebrate the periodic table? --Justin, Johannesburg, South Africa
Answer: The periodic table offers a way of organizing the natural elements which make up our universe in a way that allows us to impose order on seeming chaos. Without the elements which comprise the periodic table, everything, from the universe in which our planet resides to the computer you’re reading this on and even you yourself, would be quite impossible! Both aesthetically and from a learning standpoint, that makes the periodic table one of the most important scientific tools ever created, which is why we at Luciteria are very proud of our efforts to make it just a little more fun and interactive for students and enthusiasts of all ages!
Question: You said smoke detectors emit radiation. Why? --Meaghan, Cork, Ireland
Answer: Smoke detectors frequently include the radioactive isotope americium-241, which gives off tiny amounts of radiation and permits the detector to function properly. The amount of americium-241 in a commercial smoke detector gives off about 1/100 of a millirem (a measure of radiation exposure) per year. By comparison, you get about 360 millirems per year simply by going about your daily life because of the normal background radiation in your environment! Pack-and-a-half-a-day smokers have been shown to get as much as 8,000 millirems, or as much radiation exposure as 300 X-rays of the chest, per year because of polonium-210, radium and radioactive lead isotopes which build up in the soil both due to natural origin and fertilizers used to boost crop yield. This makes Luciteria’s element samples useful for health and anatomy classes as well as the so-called “harder” sciences!
Question: How much do Luciteria element samples cost? --Shanice, Seattle, WA
Answer: Each element is different. The prices are based on availability and ease of sourcing. Harder-to-find element samples will cost more than commonly available ones, and the format in which you order them makes a difference as well. Lucite blocks are typically less expensive than functional reference density blocks or refined elemental metal samples because they are less labor-intensive. For the latest prices and to see which elements are currently available, we encourage you to visit our website regularly!
Question: What do I need to get started in element collecting? --Erica, Point Barrow, AK
Answer: All you really need is an interest in the natural world and the willingness to do some research and exploration on your own. As we mentioned above, many collectors don’t stop at just the elements themselves, but also collect and display items which incorporate the elements as well. A good sense of curiosity and natural wonder will take you far in this fun and exciting hobby!
Question: Can I give Luciteria element samples as gifts? --Kelly, El Paso, TX
Answer: Absolutely! Luciteria element samples make wonderful presents for birthdays, Hanukkah, Christmas, graduations, anniversaries, and much more. If you’re looking for something truly unique to give the natural enthusiast in your life, a Luciteria element sample is a great, unique way to give a gift even the hardest person to buy for is sure to appreciate!
Question: What elements does Luciteria offer? --Jonathan, Paso Robles, CA
Answer: Luciteria’s complete inventory of elements in Lucite blocks includes the following. If you’re looking for functioning reference density blocks, the availability and elements may differ somewhat. Here’s our current Lucite element sample display lineup:
Actinium
Aluminum
Americium
Antimony
Argon
Arsenic
Barium
Beryllium
Bismuth crystal
Boron
Bromine
Cadmium
Calcium
Carbon
Cerium
Cesium
Chlorine
Chromium
Cobalt
Copper
Dysprosium
Erbium
Europium
Fluorine
Francium
Gadolinium
Gallium
Germanium
Gold
Hafnium
Helium
Holmium
Hydrogen (h2)
Hydrogen (h3--tritium)
Indium
Iodine
Iridium
Iron
Krypton
Lanthanum
Lead
Lithium
Lutetium
Magnesium
Manganese
Mercury
Molybdenum
Neodynium
Neon
Neptunium
Nickel
Niobium
Nitrogen
Osmium
Oxygen
Palladium
Phosphorus
Platinum
Potassium
Praseodymium
Protactinium
Radium
Radon
Rhenium
Rhodium
Rubidium
Ruthenium
Samarium
Scandium
Selenium
Silicon
Silver
Sodium
Strontium
Sulfur
Tantalum
Technetium
Tellurium
Terbium
Thallium
Thorium
Thulium
Tin
Titanium
Uranium (depleted)
Vanadium
Tungsten
Xenon
Ytterbium
Yttrium
Zinc
Zirconium
Please note that at any given time, some elements, particularly rare earth metals, Noble metals and transition metals, may be sold out due to demand. We are pleased to help collectors complete their element collection by advising them when harder-to-source elements are available again.